Quick Answer: Pure terpenes are legal to import and sell in Germany if they contain less than 0.3% THC and comply with EU quality and safety regulations. Importers must provide full documentation, including a certificate of analysis, REACH registration, and CLP-compliant safety data sheets.
Federal law permits terpene use across cosmetics, food, and industrial applications, though states like Bavaria enforce stricter standards. Companies must maintain EU-GMP quality systems, German-language documentation, and traceable sourcing. Proper compliance ensures seamless customs clearance, market access, and alignment with BfArM and European Pharmacopoeia standards for terpene-containing products.

Key Takeaways
- Pure terpenes containing less than 0.3% THC are fully legal in Germany under the Medical Cannabis Act and exempt from narcotic control.
- Importing terpenes requires a full documentation package: ISO 17025 COA, EU-GMP or equivalent certification, EORI registration, CLP-compliant safety data sheet in German, and an accurate HS code (3301).
- Bavaria enforces the strictest standards with zero THC tolerance and extra documentation, while Berlin, Hamburg, and NRW adopt more business-friendly interpretations.
- All terpenes sold in Germany must meet pharmaceutical-grade testing: GC-MS profiling, pesticide and heavy metal screening, and microbiological analysis to EU Pharmacopoeia limits.
- Hemp-derived terpenes are legal if extracted from certified seeds and remain below 0.3% THC; synthetic and botanical terpenes also qualify when properly labeled.
- Partnering with Terpene Belt Farms ensures compliant COAs, EU-ready documentation, and full support for BfArM and REACH requirements for smooth entry into the German market.
Germany represents one of Europe’s largest markets for terpene procurement, with established supply chains serving cannabis formulators, vape manufacturers, and hemp product developers across all 16 federal states.
Whether you’re sourcing cannabis-derived terpenes for high-potency formulations or botanical alternatives for mainstream CPG applications, German importation requires specific documentation: BfArM notifications, CLP-compliant safety data sheets, and proper REACH registration through your ECHA representative.
The distinction between isolated terpenes and full-spectrum preparations directly impacts your regulatory pathway, particularly when differentiating between flavoring substances and cosmetic ingredients.
This guide breaks down the exact compliance requirements for terpene procurement in Germany, from establishing your EORI number to working your way through state-specific enforcement variations that affect wholesale operations throughout the country.
German Federal Terpene Laws: What’s Legal in 2025
Pure terpenes containing no detectable THC are exempt from narcotic regulations in Germany. The Federal Institute for Drugs and Medical Devices (BfArM) classifies them as aromatic compounds suitable for industrial use, which creates clear procurement pathways for product developers.
The Medical Cannabis Act (MedCanG) established that products containing terpenes must demonstrate THC levels below 0.3% to avoid being classified as controlled substances. This threshold applies to all terpene applications, including cosmetics, food flavoring, and aromatherapy. Licensed medical cannabis producers routinely specify terpene content, with most emphasizing “unaltered terpene profiles” as quality markers.
Industrial hemp regulations under Section 1(9) KCanG provide more clarity. Hemp-derived products for commercial purposes are exempt from cannabis restrictions if the THC level is below 0.3% and are derived from certified seeds. This supports terpene extraction from compliant hemp, creating a legal foundation for hemp-derived terpene production.
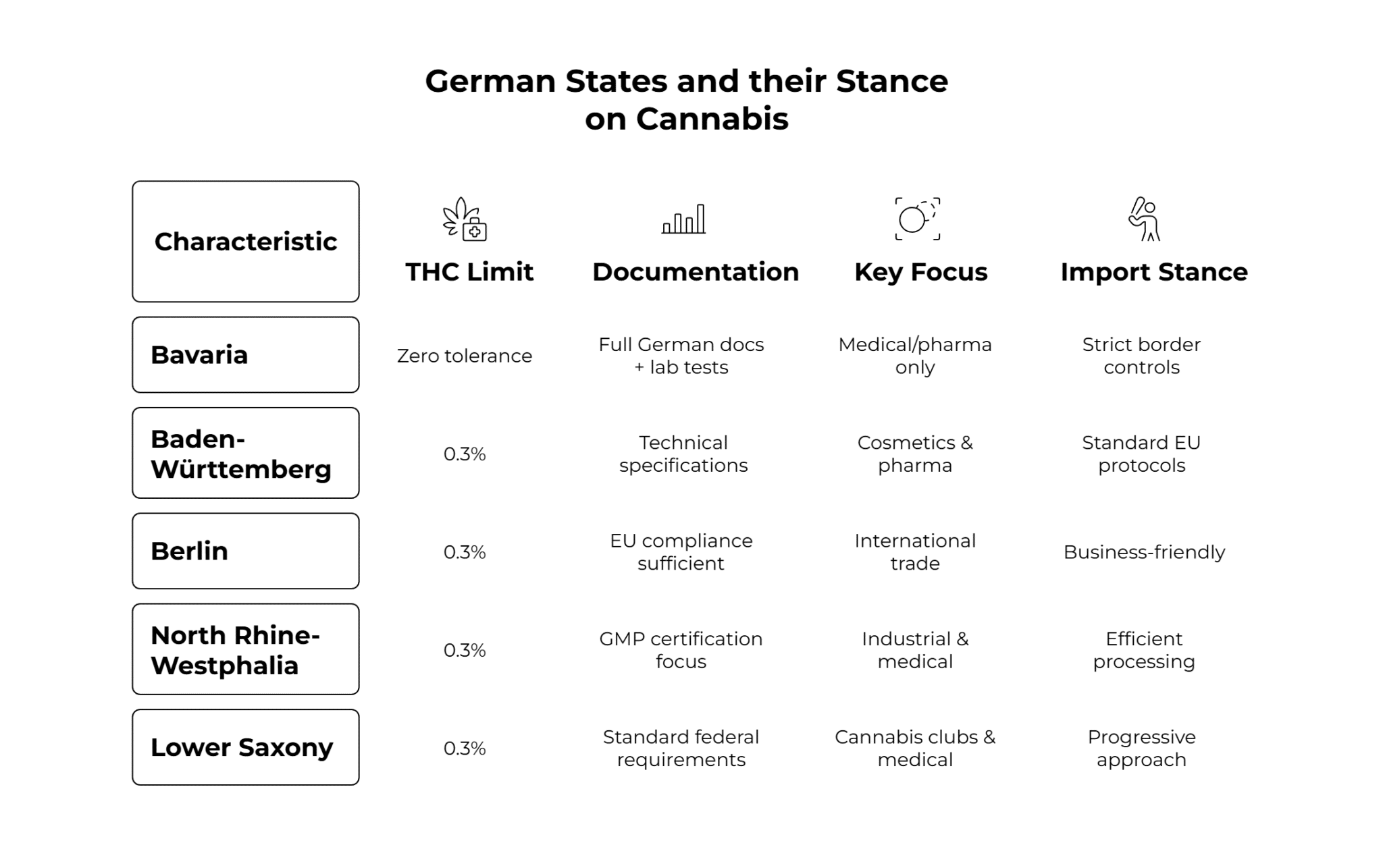
State-by-State Compliance Requirements
While the regulations for various states are practically the same, some exceptions, like Bavaria, are stricter than others.
Bavaria: Germany’s Strictest Enforcement
Bavaria maintains the most conservative approach to cannabis-related products. The state’s opposition to cannabis liberalization means heightened documentation requirements and increased border controls.
For terpene compliance in Bavaria, you’ll run into various barriers:
- Extensive German-language documentation
- Zero tolerance for THC
- Enhanced scrutiny at border crossings
- Products must avoid cannabis marketing associations
- Certificates of analysis from accredited labs are required
Baden-Württemberg
Baden-Württemberg balances business-friendly policies with thorough safety standards. Home to major pharmaceutical companies and Stuttgart’s cosmetics industry, the state represents a critical market for quality terpenes.
The state permits terpene use in cosmetics under Regulation (EC) No 1223/2009, with additional requirements for therapeutic claims. Successful suppliers provide detailed technical data sheets specifying composition, purity levels, and application rates. Baden-Württemberg’s position between Swiss, French, and German markets means authorities often adopt the most stringent requirements from neighboring jurisdictions.
Progressive Northern States
Berlin, Hamburg, and North Rhine-Westphalia maintain more liberal interpretations of federal law. These states serve as commercial hubs where international suppliers establish German operations. Lower Saxony leads in cannabis club licensing with nine associations already operational, signaling openness to terpene-enhanced products within legal boundaries.
Eastern states like Saxony and Thuringia support hemp cultivation for industrial purposes, potentially developing domestic terpene production. These regions prioritize agricultural diversification through hemp-derived product development.
Import Documentation Checklist
Importing terpenes into Germany requires specific documentation depending on the source and intended use. Companies must prepare detailed paperwork before shipment to avoid customs delays or rejection.
- Certificate of Analysis: From an ISO 17025 accredited laboratory confirming <0.3% THC
- EU-GMP Certificate: Or equivalent quality standard documentation
- EORI Registration: European customs registration for the importing entity
- Commercial Invoice: With accurate HS code classification (typically 3301)
- Safety Data Sheet: In the German language for workplace safety compliance
- Origin Certificate: Demonstrating source country and cultivation compliance
Additional requirements for medical or pharmaceutical applications include stability data, microbiological testing to European Pharmacopoeia standards, and heavy metal analysis. The BfArM may require proof that cannabis-derived terpenes originate from UN Single Convention compliant cultivation programs.
Transit through other EU states can trigger additional scrutiny, particularly when routing through the Netherlands or entering Bavaria. Working with customs brokers familiar with terpene classification prevents costly delays.
Quality Testing Standards
German buyers expect pharmaceutical-grade quality regardless of application. Testing requirements exceed basic compliance, reflecting Germany’s heritage of precision and safety.
- Terpene Profile Analysis: GC-MS identification and quantification of all compounds
- Residual Solvents: Meeting ICH Q3C guidelines for pharmaceutical products
- Pesticide Screening: Compliance with EU Maximum Residue Levels (MRLs)
- Microbiological Testing: European Pharmacopoeia limits for pathogens
- Heavy Metals: Lead, cadmium, mercury, and arsenic below specified limits
- THC Content: Verified below 0.3% threshold
Some buyers may request enantiomeric purity analysis to verify natural versus synthetic production. This particularly applies to high-value monoterpenes with synthetic alternatives. Certificates of analysis must be current, typically within six months of delivery.
Where German Businesses Source Terpenes
Germany’s import dependency for cannabis-derived compounds has created a huge market for terpene supply. There are multiple procurement channels that serve different business needs and volumes.
International Suppliers dominate the premium segment. Companies like Terpene Belt Farms provide California-sourced profiles with detailed documentation. These suppliers offer customization options and technical support that are definitely needed for product development in Germany.
Best Practices for Compliance in the German Market
Successfully working with Germany’s regulations requires strategic planning across legal entity structure, quality assurance protocols, and regional compliance variations that can significantly impact terpene procurement timelines and market access.
Quality Systems and Documentation
Implementing pharmaceutical-grade quality systems aligns with German regulatory expectations, particularly for products entering the medical or wellness sectors, where EU-GMP standards apply.
Digital document management systems become essential for maintaining compliance across Germany’s federal structure, where enforcement can vary significantly between states. For example, Bavaria typically maintains stricter enforcement while Berlin adopts more lenient approaches.
Extensive batch records, change control procedures, and supplier audit documentation demonstrate the commitment to quality that echoes strongly with German business culture’s emphasis on precision and reliability.
Regulatory Expertise
Working with German regulatory consultants provides invaluable market intelligence for dealing with the subtle contradictions between federal regulations and state-level enforcement.
These specialists help interpret changing requirements, understand regional enforcement variations, work through supplier audits, and provide early warning of regulatory changes that could impact terpene importation or distribution. Germany follows strict pharmacopoeial standards, including specific requirements for terpene content in cannabis products and detailed quality specifications.
Closing Thoughts — Book Your Terpene Supply
Ready to enter the German market with compliant terpene solutions? Terpene Belt Farms provides comprehensive documentation meeting German regulatory requirements for seamless importation and distribution.
Here’s what we offer for the German market entry:
- Fresh Never Frozen® Cannabis Terpenes: Steam-distilled and cannabinoid-free profiles available in Exclusive, Premium, and Standard tiers, each with complete analytical data
- Water-Soluble Terpene Formulations: Ideal for beverage applications and wellness products requiring EU-compliant ingredients
- Flower Infusion Solutions: Specialized formulations for enhancing botanical products while maintaining regulatory compliance
- Professional Sample Kits: Complete testing packages with technical documentation aligned to German pharmaceutical standards
Every profile includes certificates of analysis, stability data, and application guidance meeting international quality standards. Our technical team provides formulation support and regulatory documentation to facilitate BfArM notifications and state-level compliance requirements.
Request our terpene samples to see if our profiles match your specific applications. For scalable solutions and volume pricing, enroll in our Wholesale program.
Frequently Asked Questions
Are Terpenes Legal to Buy and Import into Germany?
Pure terpenes are legal in Germany as long as they contain less than 0.3% THC and meet EU quality standards. The 2024 Cannabis Act removed cannabis from the BtMG narcotics list, clarifying that pure terpenes without THC or controlled cannabinoids are not classified as narcotics. They can be purchased for product formulation, industrial use, and various applications, including cosmetics, food flavoring, and aromatherapy.
What Documentation Do I Need to Import Terpenes into Germany?
Import documentation for the German market includes:
- Certificate of Analysis from an ISO 17025 accredited laboratory confirming <0.3% THC
- EU-GMP certificate or equivalent quality standard documentation
- EORI registration for European customs
- Commercial invoice with accurate HS code classification (typically 3301)
- German-language safety data sheet for workplace compliance
- Origin certificate demonstrating source country compliance
Additional requirements may apply for medical or pharmaceutical applications, including stability data and microbiological testing to European Pharmacopoeia standards.
Do Different German States Have Varying Requirements for Terpenes?
Yes, German states interpret federal regulations differently. Bavaria maintains the strictest enforcement with zero tolerance for THC, mandatory German-language documentation, and enhanced border controls. Baden-Württemberg balances innovation with oversight, requiring detailed technical specifications for cosmetics applications. Progressive northern states like Berlin, Hamburg, and North Rhine-Westphalia maintain more liberal interpretations, focusing on EU compliance and business-friendly approaches.
What Quality Testing Standards Must Terpenes Meet for the German Market?
German buyers expect pharmaceutical-grade quality with mandatory testing, including:
- Terpene Profile Analysis using GC-MS identification
- Residual Solvents meeting ICH Q3C pharmaceutical guidelines
- Pesticide Screening compliant with EU Maximum Residue Levels
- Microbiological Testing to European Pharmacopoeia standards
- Heavy Metals analysis (lead, cadmium, mercury, arsenic)
- THC Content verification below 0.3% threshold
Some buyers may also request enantiomeric purity analysis to verify natural versus synthetic production. Certificates of analysis must be current, typically within six months of delivery.
Sources Used for This Article
- CMS Law: “Cannabis law and legislation in Germany” – cms.law/en/int/expert-guides/cms-expert-guide-to-a-legal-roadmap-to-cannabis/germany
- EUR-Lex: Regulation (EC) No 1223/2009 of the European Parliament – eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32009R1223
- Genesis Global: “Navigating Pharmaceutical Regulations in Germany” – generisonline.com/navigating-pharmaceutical-regulations-in-germany-a-comprehensive-overview/






